What was the ‘Elephant Man’ drug testing trial, what is TGN1412 and what happened to the men involved?
Volunteers left writhing in agony and projectile vomiting before their immune systems crashed and they suffered multiple organ failure

A CATASTROPHIC clinical test that left six volunteers fighting for life in intensive care became notorious as the "Elephant Man" drug trial because of the horrific side-effects they endured.
Potential wonder cure TGN1412 left the men writhing in agony and projectile vomiting before their immune systems crashed and they suffered multiple organ failure.
Now almost 11 years on the victims and the doctors who saved their lives have recalled the dramatic events of March 2006 for a new BBC documentary.
So what went wrong, who was to blame and what happened next for the human guinea pigs?
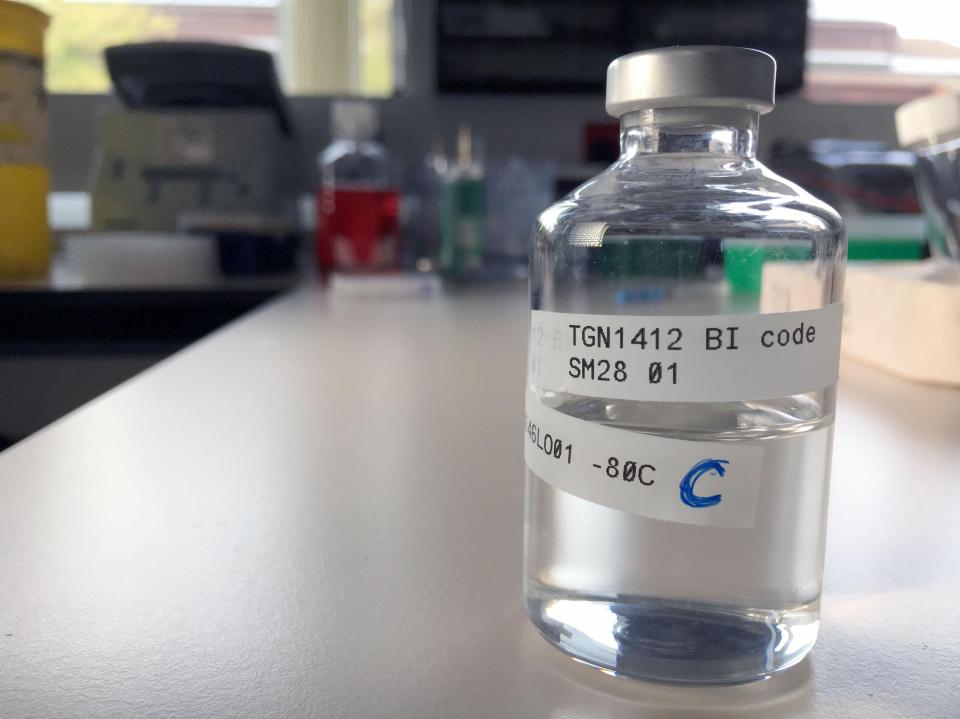
What is trial drug TGN1412?
TGN1412 was the trial name for a type of monoclonal antibody therapy manufactured by German firm TeGenero.
It was meant to manipulate the body's immune system to treat leukaemia and auto-immune diseases such as arthritis.
Researchers had high hopes for it after macaque monkeys injected with it showed no problems.
The next step was to test it on human guinea pigs in a so-called Phase 1 trial.
What was the Elephant Man drugs trial?
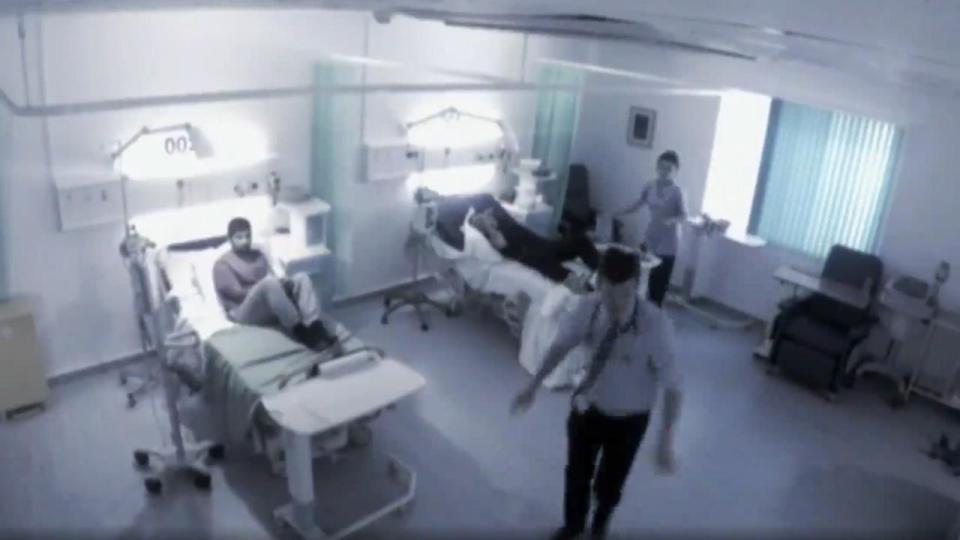
In March 2006 eight healthy volunteers were selected to take part the Phase 1 trial of TGN1412, organised by the US company Parexel.
The men aged 19 to 34 were each paid £2,000 to be given the drug in a private unit at Northwick Park Hospital in North-West London.
They were all hooked up to drips in a ward with two rooms, and then at two-minute intervals nurses injected each man with the experimental fluid.
The drug was administered ten times faster than it had been in monkeys.
In fact only six of the men were given TGN1412 while two had a harmless placebo - but neither they nor the medical staff knew who got what.
It soon became obvious. The lucky two who had a dummy drug watched in horror as the other six volunteers suddenly became very ill.
What happened to the patients in the Elephant Man drugs trial?
Within minutes, the volunteers started to "feel cold" and suffered excruciating pain in their heads and backs. Within hours medics were panicking as one by one the patients collapsed.
Student Nav Modi, then 24, of East London, said it felt like his "brain was on fire", adding: "I felt my head swelling up like an elephant's - I thought my eyeballs were going to pop out."
He told the Sun as he recovered weeks later: "I was suddenly gripped by a pain I can barely begin to describe.
"Then somehow the pain got even worse with the pressure in my head so intense it was like a truck had been parked on it."
He was told to "lie down" but the pain moved to his back.
He said: "I felt even worse than before and I was conscious of bucking and writhing in the bed as they tried to get an oxygen mask on me.
"It felt like a terrible nightmare."
Recent graduate Raste Khan, 23, had a placebo but feared he would suffer the same terrifying side-effects as the men around him.
He said: "It was all manic, everything was happening all at once. They were vomiting, they were screaming in pain, people were fainting, they couldn't control their bowels.
"It was like a horror movie."
Rob Oldfield, then 31 and just back from an acting course in Los Angeles, said almost immediately after being given the drug it felt as though he had been "dipped in ice"
He told the BBC documentary: "My whole body went freezing cold and I started shaking.
"This wasn’t something you could stop, it was so extreme. It was horrendous."
New Zealander David Oakley's head swelled so much it looked like a ball with slits - giving rise to the Elephant Man tag.
His fiancee Katrina, who rushed to his bedside in intensive care at 3.30am, recalls: "I don’t think anything could prepare you for what you saw when you first went in.
"His cheeks were very swollen, so much so that his eyes looked more like slits. His face was just round like a ball and his stomach was huge.
"It was pretty scary to see somebody you love so disfigured."
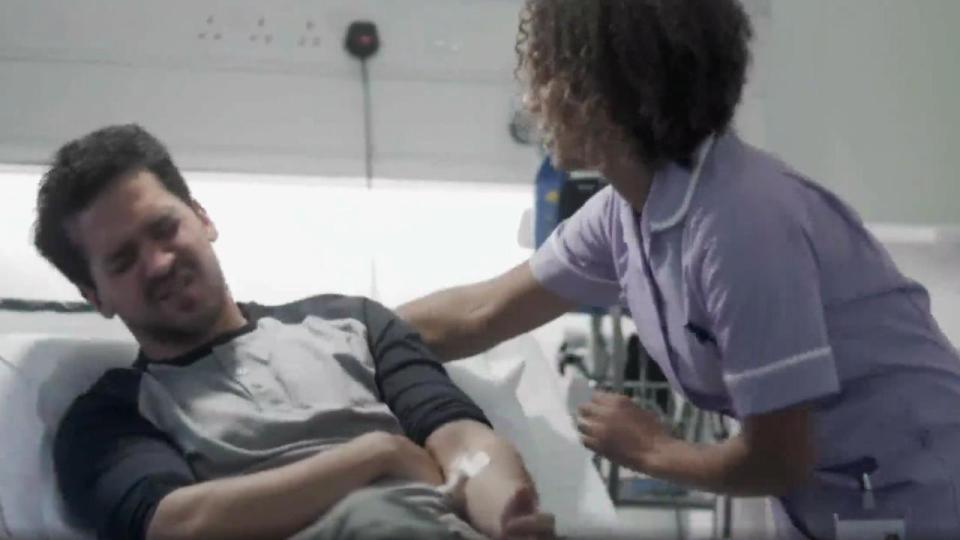
The patients had suffered an extreme immune response called a cytokine storm. They were given huge doses of steroids and kept alive by machines as their vital organs shut down.
Doctors did not know if they would survive and told relatives to say their goodbyes.
What happened next for the Elephant Man trial patients?
The worst affected was trainee plumber Ryan Wilson, then 21, who almost died after a devastating immune reaction left him with heart, liver and kidney failure.
When he woke from his coma two and a half weeks later, doctors told him: "You should be dead."
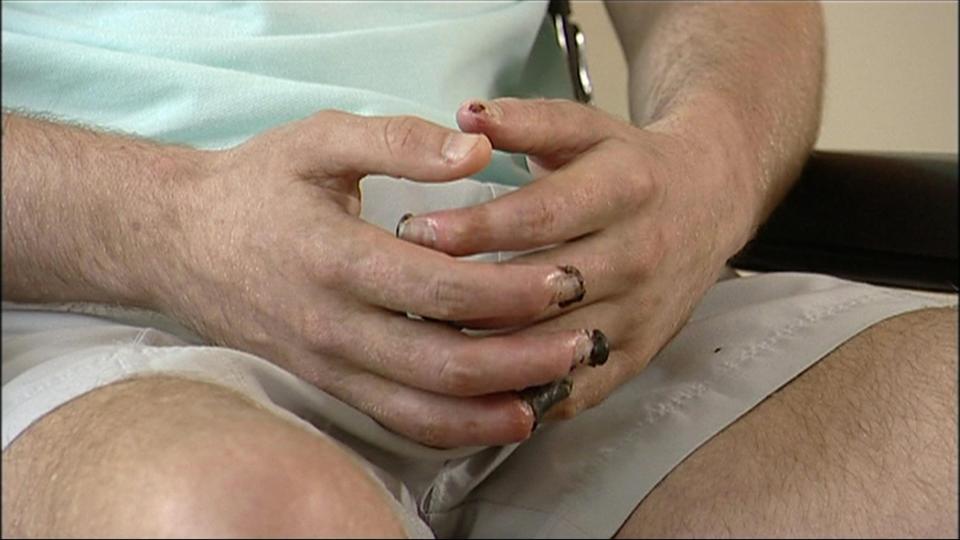
He spent four months in hospital with pneumonia, septicaemia and dry gangrene which meant several fingertips, all his toes and part of his foot had to be amputated.
Years later he still could not walk unaided despite several operations to repair skin that would not heal.
Others were able to leave hospital after three weeks, but were left shockingly weak and vulnerable to deadly infections.
David, who signed up to the trial to help pay for his wedding, said: "My organs were back working, but I was like an 80-year-old, my muscles were wasted away.
"We had no immune system at all, we were given instructions not to go on the Tube, trains or buses in case someone was to cough near us."
The six victims still do not know what the long-term damage to their bodies will be and they face a lifetime of uncertainty after warnings they could be at higher risk of cancer and other illnesses.
What happened after the Elephant Man trial and did anything change because of it?
German drug manufacturer TeGenero went bust after the disastrous first human trial of its supposed wonder cure TGN1412.
It emerged the firm had inadequate insurance, for a total of only £2million - leaving the victims in limbo as the company that conducted the trial, Parexel, at first refused to accept joint liability.
Parexel later reached a settlement and paid undisclosed compensation.
An investigation by the Medicines and Healthcare Products Regulatory Agency two months after the trial found there was no provision for 24-hour medical care.
Researchers had also not properly considered the safe dose for humans before giving it to the volunteers, and the drug was administered ten times more quickly into their bloodstream than it had been in monkeys.
The MHRA’s probe paved the way for a further independent report that made 22 recommendations to improve the safety of Phase 1 trials — including that volunteers should not be dosed all in the same day.
The guidelines now apply throughout Europe.
Two years ago it was revealed TGN1412 was making an astonishing comeback as a potential treatment for arthritis.
The rights were bought by Russian company TheraMAB which renamed it TAB08.
Trials were once again being conducted on humans - but at just five per cent the dose used in the Elephant Man tests.
READ MORE
Human guinea pigs disfigured by ‘Elephant Man drug trial’ tell of limb loss horror